Abstract
Background: Maintenance therapy has garnered much interest in multiple myeloma (MM) owing to the incurable nature of the disease. Maintenance therapy has so far been shown to improve progression-free survival (PFS), but not always overall survival (OS). However, the benefit in the Asian population in the real world remains unknown. In real world practice, implementation of maintenance therapy is heterogeneous and often based on physician choice in Asia.
Method: We included newly-diagnosed MM patients who were treated with novel agent-based induction therapy, with complete maintenance, transplantation and ISS data from year 2002 in our institution. We divided them into transplant eligible and ineligible cohorts. For each cohort, we evaluated the difference in OS, progression-free survival to first relapse (PFS1), and progression-free survival to second relapse (PFS2) between patients with and without maintenance therapy. We further evaluated if certain subgroup of patients would benefit more from maintenance therapy. Lastly, we evaluated if there was any difference in OS, PFS1, and PFS2 for patients according to the types of immunomodulatory drugs (IMiDs) used for maintenance therapy.
Results: Among the 247 evaluable patients, 56 (22.6%) patients received maintenance therapy and 191 patients did not. The maintenance therapies received included thalidomide (n=31), lenalidomide (n=18), and bortezomib (n=7). We included patients from all ISS groups: ISS stage I (n=40), ISS stage II (n=98), and ISS stage III (n=109); and both transplant eligible (n=123) and ineligible (n=124) patients. All patients received novel agents for induction therapy, including proteasome inhibitor (n=72), immunomodulatory agent (n=92), and both proteasome inhibitor and immunomodulatory agent (n=83).
For the transplant eligible patients, maintenance therapy was given for 33.3% of the ISS stage I patients, 34.0% of the ISS stage II patients, and 16.3% of the ISS stage III patients. On the other hand, for the transplant ineligible patients, maintenance therapy was given for 12.5% of the ISS stage I patients, 14.5% of the ISS stage II patients, and 23.3% of the ISS stage III patients.
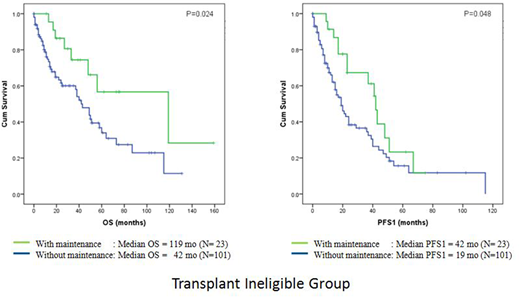
Our study showed that for the transplant eligible patients, maintenance therapy did not result in any significant difference in OS, PFS1, or PFS2 (P=0.069, 0.685, 0.350 respectively). However, for the transplant ineligible patients, there was a significant improvement in OS (median OS 119 mo vs 42 mo; P=0.024) and PFS1 (median PFS1 42 mo vs 19 mo; P=0.048), but not PFS2 (P=0.373), with maintenance therapy. Subgroup analysis showed that the OS benefit was mainly seen in the ISS stage III group (median OS 119 mo vs 38 mo; P=0.017) and there was a trend towards PFS1 benefit (median PFS1 41 mo vs 16 mo; P=0.060) with maintenance therapy. We combined the ISS stage I and II patients as the lower risk group for evaluation and our evaluation did not show any OS, PFS1 or PFS2 benefit of maintenance therapy in this cohort (P=0.373, 0.292, 0.251 respectively). We did not see any difference in OS, PFS1, and PFS2 (P=0.444, 0.170, and 0.512 respectively) whether patients had thalidomide, or lenalidomide, or no maintenance.
Conclusion: Our study in this Asian population shows that maintenance therapy is not routinely given in clinical practice. For the transplant eligible patients, there is a trend towards more maintenance therapy given for the lower risk group patients, while in the transplant ineligible patients, maintenance therapy tends to be given for the higher risk group patients. However, the percentage remains small (less than 40%) in all groups. Maintenance therapy has OS and PFS1 benefit for the transplant ineligible patients, especially for the higher risk group patients. There is, however, no OS, PFS1, or PFS2 benefit observed for the transplant eligible patients. We plan to expand our study by collaborating with other Asian institutions to evaluate more patients.
Chng:Takeda: Consultancy, Honoraria, Other: Travel, accommodation, expenses; Janssen: Consultancy, Honoraria, Other: Travel, accommodation, expenses, Research Funding; Amgen: Consultancy, Honoraria, Other: Travel, accommodation, expenses; Aslan: Research Funding; Merck: Research Funding; Celgene: Consultancy, Honoraria, Other: Travel, accommodation, expenses, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal